Struttura: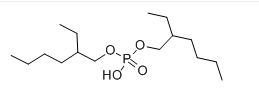
| Dehra | Likwidu bla kulur sa kemmxejn isfar ċar |
| Formula | C16H35O4P |
| CAS Nru. | 298-07-7 |
| KE Nru. | 206-056-4 |
| UN NO. | 1902 |
| KODIĊI HS | 2919900020 |
| Kontenut (%) | ≥95 |
| Densità (20 ℃) g/ml | 0.9736 |
| Acid | 159-189 |
| Flash Point℃: | ≥160 |
| Sinonimi | Bis(2-etileżil) phosphate |
D2EHPA is soluble in organic solvents such as kerosene, aliphatic hydrocarbons, and aromatic
hydrocarbons. This solubility allows for the formation of organic phases in solvent extraction
systems,enabling the separation of metal ions from aqueous solutions.
D2EHPA exhibits a high extracting power for a wide range of metal ions, including rare earth elements,
uranium, copper, nickel, and cobalt. It selectively forms complexes with these metal ions, allowing for
their efficient extraction and separation from other impurities.
D2EHPA is an acidic extractant due to the presence of the phosphoric acid group. It can protonate metal
ions in the aqueous phase and form water-insoluble metal-D2EHPA complexes, which can be easily separated
from the aqueous solution.
D2EHPA forms stable complexes with metal ions, enabling the extraction and subsequent recovery of metals.
These complexes are relatively resistant to hydrolysis, which ensures their stability during the extraction process.
D2EHPA is considered toxic and should be handled with care. It can cause skin and eye irritation and should
not be ingested. Proper safety precautions, such as the use of protective equipment, should be followed when
working with D2EHPA.
D2EHPA is susceptible to degradation by strong acids and bases. Exposure to acidic or alkaline conditions can
lead to the hydrolysis of D2EHPA and the loss of its extracting properties. Therefore, the pH conditions should
be controlled in solvent extraction processes involving D2EHPA.
D2EHPA’s extracting power can be influenced by temperature. Generally, higher temperatures enhance the
extraction efficiency of D2EHPA. Madankollu, excessive temperatures can also lead to the degradation of
D2EHPA, impacting its performance.



D2EHPA is widely used as an extractant in solvent extraction processes for the separation and recovery
of metals. It is particularly effective in extracting rare earth elements, uranium, copper, nickel, cobalt,
and other metal ions from aqueous solutions. D2EHPA forms complexes with these metals, allowing for
their selective extraction and subsequent purification.
D2EHPA is extensively employed in the extraction of rare earth elements from their ores or concentrates.
It is used to separate REEs from other impurities in a solvent extraction circuit. D2EHPA’s high selectivity
for REEs enables the efficient recovery and purification of these valuable elements, which are crucial for
numerous technological applications.
D2EHPA plays a vital role in the extraction and purification of uranium, a key fuel material in nuclear
power generation. It is employed in the separation of uranium from other elements in the ore processing
stage. D2EHPA forms stable complexes with uranium ions, enabling their efficient extraction and
subsequent recovery.
D2EHPA is utilized for the purification of various metals obtained through hydrometallurgical processes.
It helps in separating target metals from impurities, such as other metal ions or radioactive elements.
.D2EHPA’s selective extraction properties aid in obtaining high-purity metals suitable for further industrial
use.
D2EHPA is also employed in the recycling of metals from industrial wastes, scrap materials, and electronic
waste. It facilitates the extraction and separation of valuable metals, allowing for their recovery and reuse,
thereby contributing to resource conservation and sustainability.
D2EHPA is utilized in analytical chemistry as a reagent for the determination of metal ions in aqueous
solutions. It can form colored complexes with certain metal ions, enabling their quantitative analysis
through spectrophotometric or colorimetric methods.
The two primary raw materials required for D2EHPA production are phosphorus pentoxide (P2O5) u
Isooctanol. These materials need to be prepared and handled according to safety guidelines.
In a reaction vessel, phosphorus pentoxide (P2O5) is added, followed by the addition of Isooctanol.
The reaction takes place under controlled conditions, typically with heating and stirring. The esterification
reaction occurs between the hydroxyl (-OH) group of Isooctanol and the phosphorus pentoxide, resulting
in the formation of D2EHPA. P2O5 + Isooctanol → Di(2-etileżil)aċidu fosforiku (D2EHPA)
During the reaction, the temperature, reaction time, and other parameters are closely monitored
to ensure the desired conversion and product quality.
After the completion of the esterification reaction, the reaction mixture is subjected to a workup
process. This typically involves neutralization of excess phosphorus pentoxide and removal of impurities
through processes such as washing, filtration, and distillation. The purification steps help in obtaining a
high-purity D2EHPA product.
The purified D2EHPA is then stored in suitable containers, following proper labeling and safety procedures.
It is typically packaged and transported for commercial distribution and use.
1. Spezzjoni deħlin: Il-materja prima ewlenija,huma spezzjonati għall-kontenut tagħhom, appearance and other main
properties.
2. Spezzjoni ta 'tmigħ: il-prinċipju ta ' l-għalf tal-materja prima huwa first-in-first-out, and the appearance of the main
raw materials is randomly inspected according to whether there is a big change in the storage conditions before
feeding.
3. Teħid ta 'kampjuni tal-lott fil-proċess tal-produzzjoni: Matul il-proċess tal-produzzjoni, the main indexes of each batch of
products: il-kontenut u l-valur tal-aċidu se jiġu eżaminati tliet darbiet f'perjodi ta 'żmien differenti.
4. Spezzjoni tal-Ħażna: Kull lott (5.6tunnellati) jiġi spezzjonat qabel il-ħażna.
5. Spezzjoni 'l barra: Skond il-kwantità mitluba mill-klijenti, the products will be sampled
and inspected.
6. Spezzjoni qabel il-ġarr: skond il-ħtieġa tal-klijent, third party inspection can be carried
out on the products before shipment.
Apparenza fiżika: Spezzjoni viżwali
Analiżi tal-Purità:Kromatografija tal-gass (GC)
Valur Aċidu:Titrazzjoni potenzjometrika
Kontenut tal-Ilma:Analizzaturi tal-umdità
Indiċi refrattiv:Rifrattometru
Densità:Miter tad-densità
Each batch of products should be accompanied by a certificate of conformity, including: the name of the
manufacturer, the name of the product, the production batch number, the net weight per barrel, the quality
level and the implementation of the standard number.weight, quality grade and implementation standard
number.
Packed in clean and dry plastic drums, net weight 200±0.3kg per drum or 1000±0.5kg, compressed and sealed
after each batch.
This product is packed in plastic drums, during transportation and loading/unloading, it should be carefully and
gently put down, and prevent from impact.
The storage place should be cool, dry and ventilated. Do fireproof and rainproof.

Il-kumpanija ssegwi l-kunċett ta ' “innovazzjoni kontinwa, l-insegwiment ta 'l-ewwel klassi”, u hija lesta li tipprovdi lill-klijenti domestiċi u barranin bi prodotti ta 'kwalità għolja u servizz sodisfaċenti.